24. lithium-doped NiO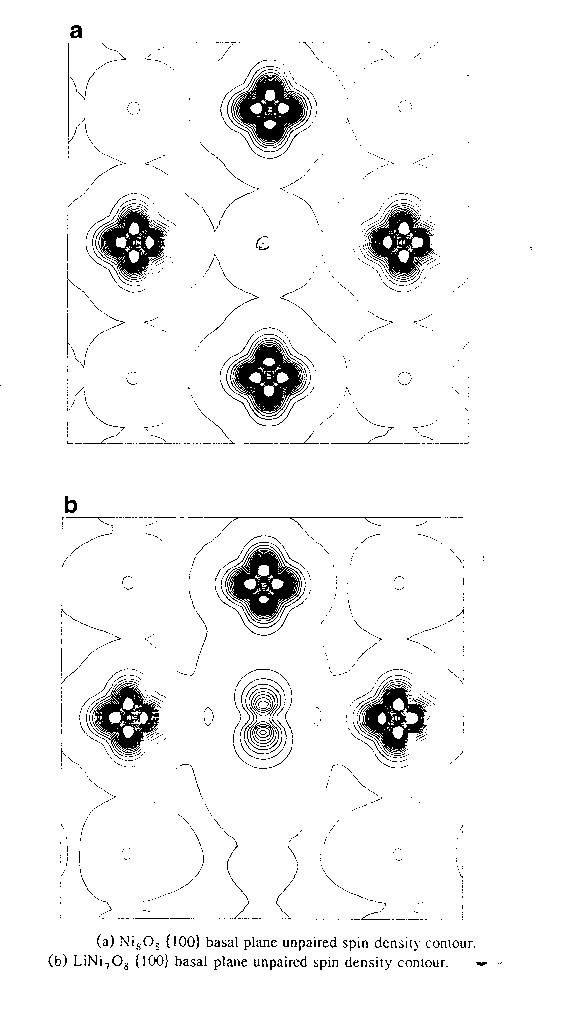 |



|
A particularly interesting question in the field of strongly-correlated materials is the following: What happened when you dope a Mott insulator with electron or hole producing impurities? The example I will focus on here is doping NiO with Li ions. Li-doped NiO is a semiconductor, because we introduce holes into the lattice, and these can move around without feeling the Hubbard U Coulomb potential. Spectroscopy results tell us that the holes are associated with the oxygen ions, rather than on the nickel sites as used to be believed (the chemistry concept of transition metal ions having 'variable valence'). We can confirm this with an electronic structure calculation. The FIRST PICTURE above is a contour plot of the spin density in undoped NiO. The isodensity contours are more widely separated than in the equivalent plot for KCuF3, but you can just about see the small spin polarization of the oxygen ions due to the superexchange. If you dope NiO with lithium, you see this enormous unpaired spin density representing the hole go onto the oxygen ion [SECOND PICTURE]. I should qualify that, because you have to watch the symmetry. If you force a fully symmetric solution, then the unpaired electron is forced to delocalize over the six oxygen ions neighbouring the Li site. If you remove that constraint, then this allows, not forces, the electronic configuration to relax to a non-degenerate state in which the hole is localized on a single oxygen site, and this is indeed what happens with a stabilisation energy of a few eV depending on the concentration of Li impurities. Now of course, if the geometry remains fully symmetric, then the many-body wave function must have the same symmetry as the crystal, so this is a broken symmetry solution. You could of course construct a fully symmetric wave function from a linear combination of localized determinants, like this one. But, we actually find this geometry is unstable with respect to distortions of this defect pair and the Li+ relaxes away from the hole towards the opposite O2. Evidently the O2- here exerts a greater attractive force on the Li+ ion compared with the O- site here. There's apparently some evidence for that in experiments as well. When you dope something with holes, and draw a band structure, you put in some states just above the valence band edge and they're called acceptor states. This is something like an empty acceptor state. The hole is currently bound to the lithium impurity, but you can imagine it takes very little thermal activation to shove an electron into here, the hole will hop over here and its no longer bound to the lithium and free to carry a current; hence this is a semiconductor. |


