25. lithium doped NiO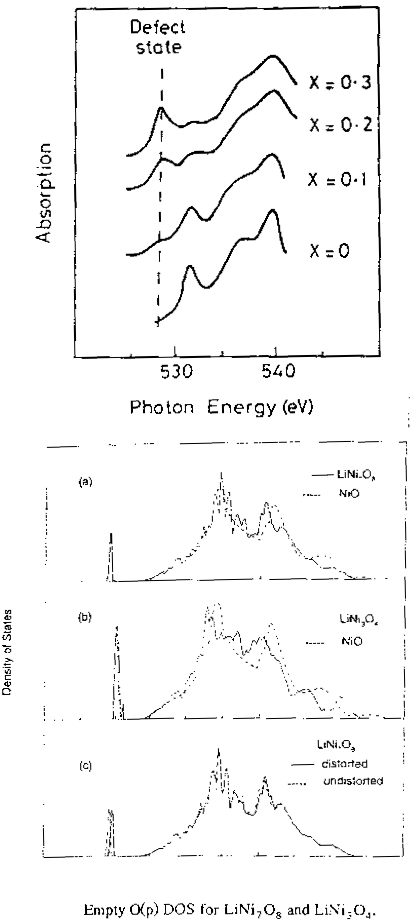 |



|
Now the first picture above is the experimental oxygen K-edge spectrum in Li doped NiO. This sort of spectrum is a signature of the empty oxygen p states. What happens is that as you dope with lithium, you get the emergence of a defect peak below the conduction band edge and this difference is close to the optical band gap of 3.7eV. So the defect peak must live just over the top of the valence band as we expect. Now in the Hartree-Fock density of states we find there is practically no change in the conduction band (dotted line NiO, solid line Li-doped NiO) but we do get this defect peak here, which correspond to additional empty oxygen p states. The difference is actually 3.5 eV, almost the same as in the spectrum. But as we know, Hartree-Fock grossly overestimates band gaps. Well, the Hartree-Fock band gap, as in the separation of the valence and conduction bands corresponds to something like a charge transfer excitation - it's the excitation energy involved in putting an extra electron onto the Ni site. But here we're comparing the energies of the defect peak and the conduction band, which both correspond to unoccupied states, so we're comparing energies of orbitals of equal occupation, so their difference in energy is something like a local ('promotion') excitation. The defect peaks must live just at the edge of the valence band, so the difference between them is quite properly an estimate of the 'gap'. OK, now for a last example, what is the effect of doping on the magnetic properties of a strongly correlated material?.. Let's look at some high Tc superconducting oxides. |


