|
So, cuprates. First of all let's just convince ourselves we're getting the
qualitatively correct ground state for one of the more famous cuprates,
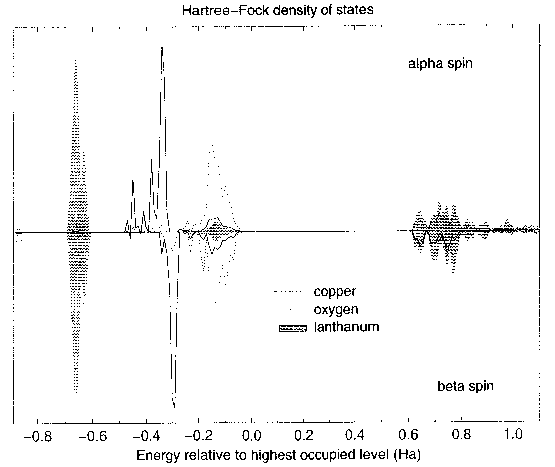
La2CuO4. In the calculated density of states above you
can see that we are. Dotted lines oxygen. Shaded peaks lanthanum. Solid lines,
total copper. Oxygen states are correctly at the top of the valence band - in
agreement with experiment. The occupied majority spin Cu states are lower in
energy than the minority spin, because of the exchange. And we have an orbital
polarization, there are unoccupied d states up here in the conduction band
(hiding under bits of lanthanum). So it's a charge transfer insulator. And if
you do the calculations, the CuO2 planes are antiferromagnetic. So everythings
really alright, and it's ready for doping. But actually I haven't bothered to do
that for this material so I'll show you another one.
|