6. Ligand field spectrum of NixMg1-xO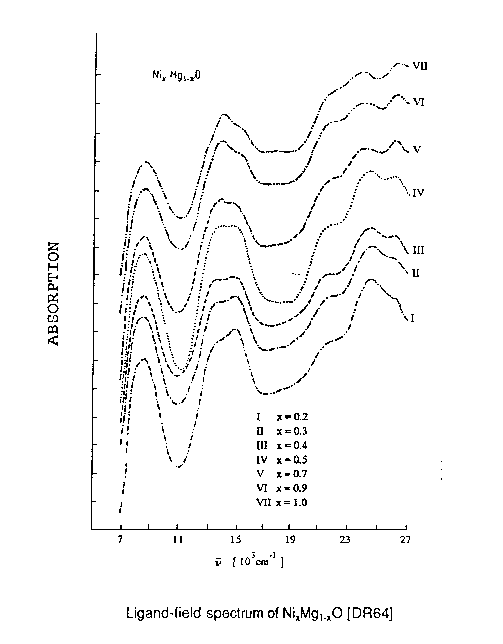 |



|
Nickel oxide and magnesium oxide have the same crystal structure and nearly the same lattice constant, so nickel can be easily substituted into MgO. The optical absorption spectrum of dilute Ni2+ in MgO has been measured [SEE PICTURE]. The significant point is that as the concentration of Ni2+ ions is increased, even all the way to pure NiO, there is hardly any change in the spectrum. It's quite clear that we are observing single ion behaviour here (modified by the crystal field) - independent ion behaviour, localized electron behaviour, whatever you want to call it, in a periodic material. You always find that this elementary picture is valid for these ions - eight of the orbitals are occupied and two are empty. This isn't the total many-body wave function of course, but it's clearly the dominant component. So Mott's insight is basically just a straightforward piece of atomic physics. The occupation of localized levels has an enormous effect on the energies of unoccupied levels, producing a gap in the one-electron band structure. This inhibits charge flow around the crystal, due to the higher interelectronic Coulomb energy arising from the presence of charge-fluctuation configurations with 9 electrons per site, as opposed to 8. |


