7. Anion states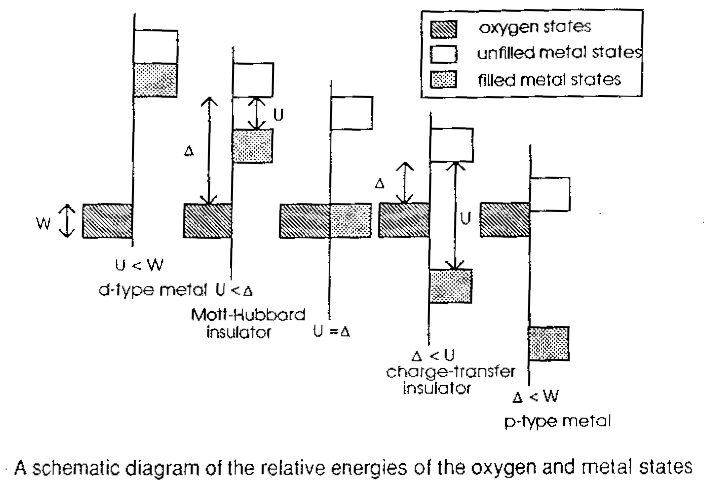 |



|
If we want to predict what the density of states should look like, it's important to remember that we need to consider the anion states (e.g. oxygen) as well. For example, NiS is a metal. Do we have to imagine that there is a huge difference in the on-site interactions between the oxide and the sulphide? No, it could just be something like the p-type metal shown in the overhead. In each of the five cases represented above, the box on the left of the line represents the anion band. On the right of each line, the grey box represents the filled states in the 3d band, the white box the unfilled states in the 3d band. In the two cases on the left, the oxygen states are way down in energy. We have a partly filled d band, so if we're on the localized side of a Mott transition we get a Mott Hubbard insulator, otherwise the d-type metal. The two cases on the right are what happens if the filled anion states are higher than the occupied 3d manifold, either a p-type metal or a charge transfer insulator. There is more or less conclusive experimental evidence that NiO is a charge transfer insulator, so the final piece in the jigsaw is that in the valence band, we expect the oxygen states to be higher than the nickel states. So we know what to expect, what do we actually get with our band theory calculation..? |


